Why is vanillin achiral?
1 Answer
Mar 10, 2016
Vanillin is achiral because it has no chiral centres, and because it has a mirror plane (coplanar with it).
Explanation:
The structure of vanillin is
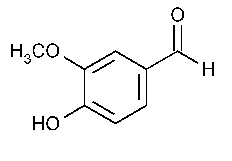
The aldehyde carbon and those in the aromatic ring are directly attached to only three other atoms. They are not chiral centres.
Only the carbon of the methyl group has four atoms attached to it, and three of those atoms are hydrogen atoms. It is not a chiral centre.
Since vanillin has no chiral centres, and it has a mirror plane (coplanar with it), it is not a chiral compound.


