Why is XeF4 molecule shape a square planer?
1 Answer
This is a consequence of
Explanation:
This is a consequence of
Around the central xenon atom there are
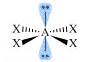
AS with all these problems, it is important to recognize that the electronic geometry is octahdedral. However, we describe the geometry on the basis of bonding pairs between atoms, not electron pairs. The geometry of
I wish I could make the illustration bigger but I am a klutz with these grafix.

