Why "Mg"^(2+) +2"e"^(-) -> "MgO" ?
From a web-tutorial on reduction potentials :
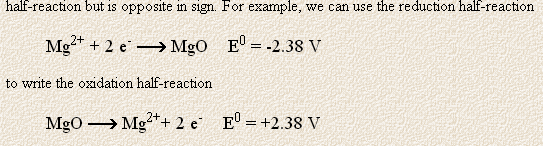
Why should adding 2 electrons to "Mg"^(2+) result in "MgO" ?
AFAIK, in "MgO" magnesium's oxidation state is also (+2) .
From a web-tutorial on reduction potentials :
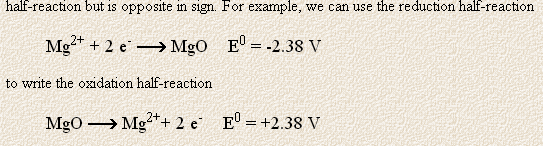
Why should adding 2 electrons to
AFAIK, in
1 Answer
That's just a typo.
Explanation:
You are right, adding two electrons to a magnesium cation,
You can oxidize magnesium metal to magnesium cations and reduce magnesium cations back to magnesium metal.
The correct reduction half-reaction would be
"Mg"^(2+) + 2"e"^(-) -> "Mg" " "E^0 = -"2.38 V"
So that is just a mistake they made when writing out the half-reaction.
For example, the synthesis of magnesium oxide,
2"Mg"_text((s]) + "O"_text(2(g]) -> 2"MgO"_text((s])
Here you have the oxidation half-reaction
2"Mg" -> 2"Mg"^(2+) + 4"e"^(-)
Each magnesium atom loses two electrons, so two magnesium atoms will lose a total of four electrons.
The reduction half-reaction is
"O"_2 + 4"e"^(-) -> 2"O"^(2-)
Each oxygen atom gains two electrons, so two oxygen atoms will gain a total of four electrons.

