Why will steam burn the skin more severely than boiling water?
1 Answer
Jul 12, 2016
Because steam is hotter than boiling water.
Explanation:
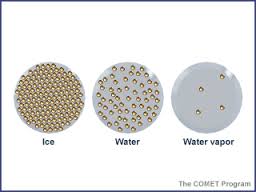
Boiling water is, very simply, water that is starting to evaporate and turn into steam - water's gaseous state.
When we look at states of matter, the gas state has more energy (expressed here as heat) than the liquid or even solid state.
As a substance gains energy, the particles that make it up gain kinetic (movement) energy and move about more. This causes them to spread out more and become less dense.
When you heat water, the water particles spread out and turn into a gas which has much more energy (and heat) than it did in liquid form.
I hope this helps; let me know if I can do anything else:)
