Will the reaction #Ag + CuNO_3# occur?
1 Answer
No. Since
This is supposed to be a redox (oxidation-reduction) reaction, where the intention is to oxidize
The half-reactions are:
#"Ag"(s) -> "Ag"^(+)(aq) + e^(-)# ,#E_"oxid"^@ = -"0.80 V"#
#"Cu"^(+)(aq) + e^(-) -> "Cu"(s)# ,#E_"red"^@' = ???#
so the reaction would hypothetically be
#color(red)("Ag"(s) + "CuNO"_3(aq) -> "Cu"(s) + "AgNO"_3)# .
WHERE IS THE REDUCTION POTENTIAL FOR COPPER(I)?
For the copper reaction, I actually can't find data for its standard reduction potential, but I can work around that.
The reference above shows the following, which we can add together:
#"Cu"^(+)(aq) -> cancel("Cu"^(2+)(aq)) + cancel(e^(-))# ,#E_"oxid"^@ = -"0.15 V"#
#cancel("Cu"^(2+)(aq)) + cancel(2)^(1)e^(-) -> "Cu"(s)# ,#E_"red"^@ = "0.34 V"#
#"--------------------------------------"#
#"Cu"^(+)(aq) + e^(-) -> "Cu"(s)# ,
#E_"red"^@' = E_"red"^@ + E_"oxid"^@#
#= 0.34 + (-0.15) = "0.19 V"#
So, the final standard cell potential for the overall redox reaction would be:
#E_"cell"^@ = E_"red"^@' + E_"oxid"^@#
#= 0.19 + (-0.80) = color(blue)(-"0.61 V")#
...a negative number.
WHAT DOES A NEGATIVE STANDARD CELL POTENTIAL MEAN?
Note the following equations, which helps us determine whether a negative
#color(blue)(DeltaG^@ = -nFE_"cell"^@)#
#color(blue)(DeltaG = DeltaG^@ + RTlnQ)# where:
#DeltaG# is the general Gibbs' free energy and#DeltaG^@# is the standard Gibbs' free energy.#n# is the number of electrons transferred in the overall reaction. In this case it is#1# .#F# is the Faraday constant, which is about#"96485 C/mol"# .#E_"cell"^@# is the standard cell potential, which we have for our reaction to be negative.
Normally,
But since standard reduction potentials are defined for
Thus, with
Now we can plug into the first equation to get
#color(green)(DeltaG = -nFE_"cell"^@)# .
Since
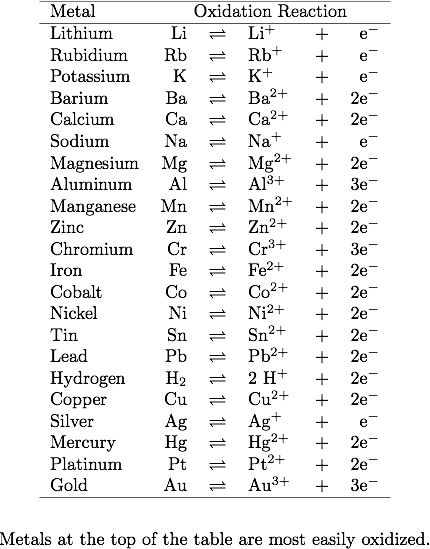
This is consistent with the activity series, which tells us that copper is more easily oxidized than silver, and thus, would rather be oxidized than reduced.

