How do catalysts affect collision theory?
1 Answer
Catalysts do not affect collision theory, but collision theory explains the effect of catalysts.
Explanation:
Collision theory states particles must collide with a certain minimum energy called the activation energy.
If there is not enough energy, the reaction will not occur.
You can mark the position of activation energy on a distribution curve like this:
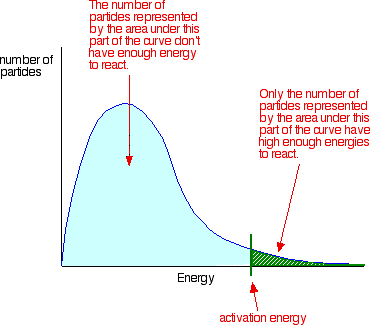
Only those particles represented by the area to the right of the activation energy will react when they collide.
To increase the rate of a reaction, you must increase the number of successful collisions.
One way of doing this is to provide an alternative way for the reaction to happen which has a lower activation energy.
In other words, you move the activation energy on the graph like this:
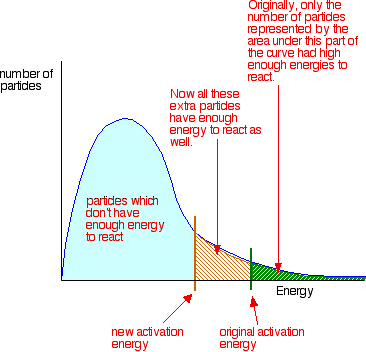
A catalyst has exactly this effect on activation energy. It provides an alternative route for the reaction. That alternative route has lower activation energy so the reaction speeds up

