What is the difference between a decomposition reaction and a replacement reaction?
1 Answer
A decomposition reaction is one where a compound is broken down into its constituent chemical species:
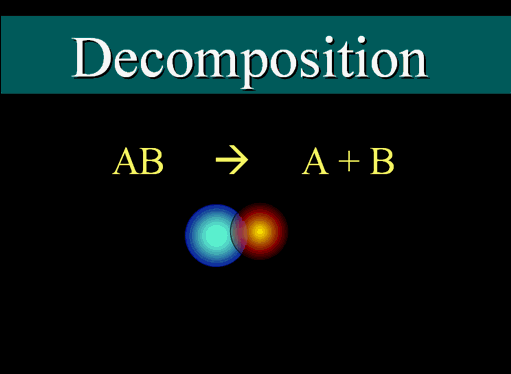
For example:
The
--(side note: the
There are two types of replacement reactions, observe the differences:
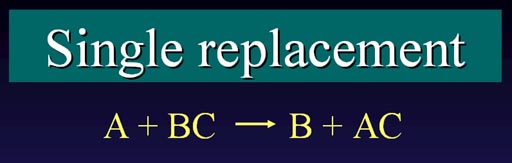
Single Replacement:
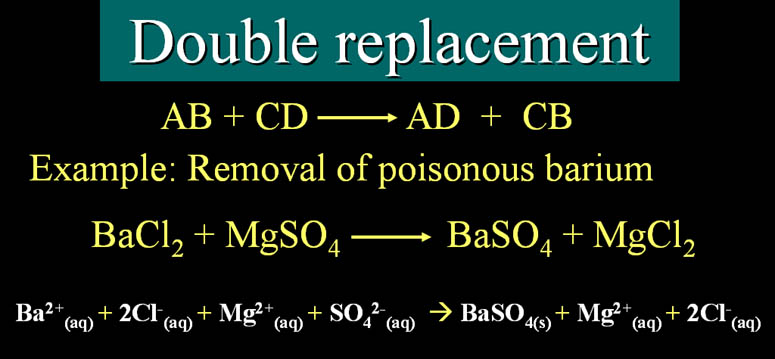
Double Replacement:

