What is an example of a VSEPR model practice problem?
1 Answer
Question: Use the VSEPR model to predict the molecular geometry of
Answer:
To predict the molecular geometry of a molecule, we first draw its Lewis structure(s) and count the number of domains around the central atom. The number of electron domains (bonding and nonbonding) gives the electron domain geometry. We can then obtain the molecular geometry from the arrangement of the domains that are due to bonding electrons.
If we draw the Lewis structure of
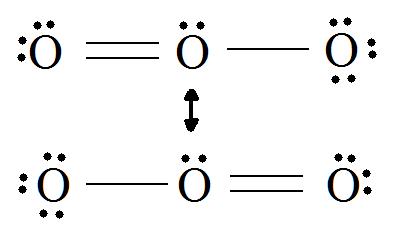
This picture shows a central oxygen with three electron domains--two bonding and one nonbonding. According to the VSEPR model, a molecule with three electron domains has a base geometry called trigonal planar. However, in the molecular geometry, one of these electron domains is nonbonding, so the derivative structure is called "bent":