What are cycloalkanes?
1 Answer
Cycloalkanes are alkanes in which the carbon atoms join to form a ring. The smallest cycloalkane is cyclopropane.
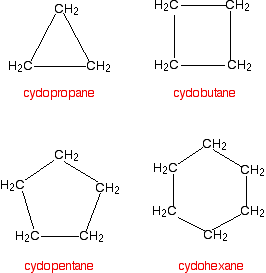
You name the cycloalkane by putting the prefix "cyclo-" in front of the name of the alkane with the same number of carbon atoms.
If you count the carbons and hydrogens, you will see that they no longer fit the general formula
The physical properties of cycloalkanes are close to those of alkanes. But they have higher boiling points, melting points, and densities than alkanes.
This is because the ring ties the carbon atoms together . The carbon atoms. cannot move around as much as in open chains.
The rings can make better contact with each other, and this increases the London dispersion forces. Cycloalkanes have higher melting points, boiling points, and densities than alkanes.
The chemical properties of cycloalkanes are like those of alkanes.
Many cycloalkanes are used in motor fuel, natural gas, petroleum gas, kerosene, diesel, and other heavy oils.


