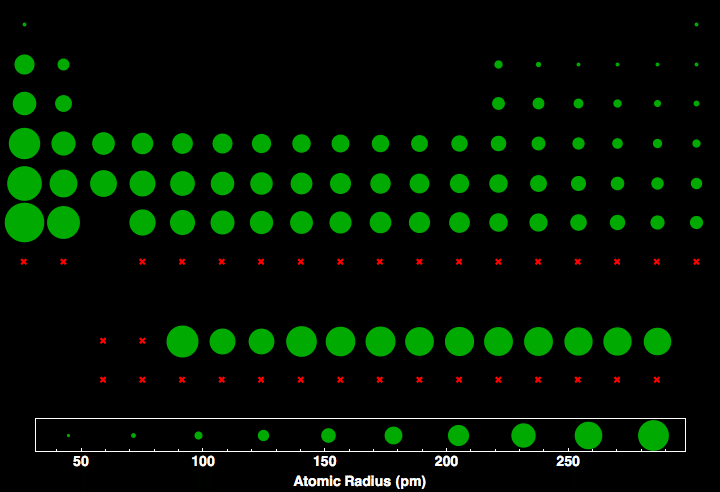
How does the atomic number affect atomic radius?
1 Answer
Jun 18, 2014
The atomic number is the amount of protons present in the atom.
Because of that, we can say that the atomic number represents the positive charge of the atom.
As the positive charge of the atom increases the atomic radius decreases because the positive charge will bring electrons closer to the nucleus.
Atomic radius becomes smaller as you move across a row/period of the periodic table because it increases the number of protons,
But, the Atomic radius becomes larger as you move down the periodic table in a group or column because it increases the number of energy levels.