How does the atomic number change when it emits an alpha particle?
1 Answer
Jun 15, 2014
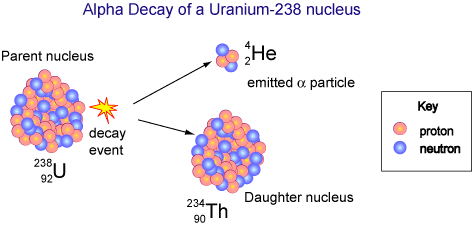
Alpha decay involves the ejection of an alpha particle from the nucleus. Since an alpha particles is a helium nucleus, it involves the loss of 2 protons and 2 neutrons.
As a result, the atom the loses an alpha particle decreases its mass number by four ( 2 protons and 2 neutrons) and decreases it's atomic number by 2 ( it loses two protons). Consequently, the identity of the element is changed to new element.
When uranium 238 undergoes alpha decay, it emits an alpha particle and the uranium is changed to thorium