How does the formation of covalent bonds relate to the octet rule?
1 Answer
Covalent bonding occurs when atoms share valence electrons to form a chemical bond. Atoms of nonmetals form covalent bonds by sharing electrons in order to achieve an octet of valence electrons, like the noble gases, except for helium, which only has two valence electrons. The octet refers to the highest energy s and p sublevels, the valence shell, which in noble gases, except for helium, are filled with eight electrons, two in the s sublevel and six in the p sublevel.
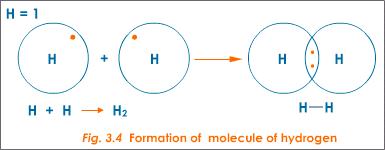
For example, hydrogen atoms each have one valence electron. Two hydrogen atoms bond covalently by sharing their single valence electrons to form a covalent bond, which forms a molecule of hydrogen gas (
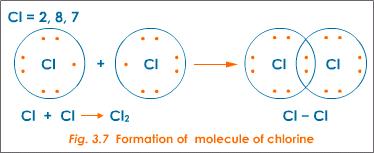
An example of atoms that bond covalently to form an octet is the formation of a covalent bond between two chlorine atoms to form a molecule of chlorine gas(
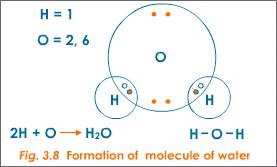
A water molecule (