How can I draw the following esters: ethyl butanoate, pentyl propanoate, propyl 3-ethylhexanoate?
1 Answer
See below.
Explanation:
Esters are alkyl alkanoates. They form during the reaction between alcohols and carboxylic acids.
The name of an ester consists of two words.
The first part of the name is the alkyl group of the alcohol.
The second part of the name is the name of the acid minus the ending –ic acid plus the ending –ate.
But we usually write the formula with the acid part first and the alcohol part second.
The acid part of the formula is the original acid minus the carboxylic
The alkyl part is
So the formula of the ester is
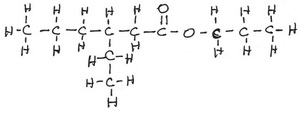
Ethyl Butanoate
Ethyl is
Butanoic acid is
The formula of ethyl butanoate then becomes
The structural formula looks like this:
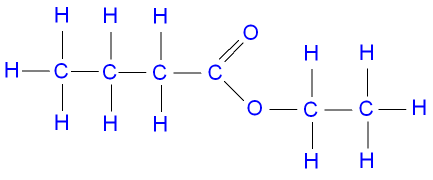
Pentyl Propanoate
Pentyl is
Propanoic acid is
The formula of pentyl propanoate then becomes
The structural formula looks like this:
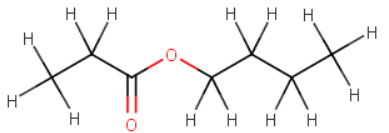
Propyl 3-Ethylhexanoate
Propyl is
Hexanoic acid is
The 3-ethylhexanoate group is
The formula of propyl 3-ethylhexanoate then becomes
The structural formula looks like this: