What are structural isomers? Give me an example
1 Answer
Structural isomers are molecules that have the same molecular formula but with the atoms connected in a different order.
There are three types of structural isomers.
1. Chain isomers
In chain isomers, the carbon atoms are connected in different orders.
There are two isomers with the formula C₄H₁₀.

In one of them, butane, the carbon atoms lie in a "straight chain".
In the other, isobutane, the chain is branched, with three C atoms in a row and the fourth attached to the central C atom.
2. Position isomers
In position isomers, the carbon skeleton remains unchanged, but functional groups are moved around.
For example, there are two structural isomers with the molecular formula C₃H₇Br.

In 1-bromopropane, the bromine atom is at the end of the chain. In
2-bromopropane, it's attached in the middle.
You can also get position isomers on benzene rings. Consider the molecular formula C₆H₄Cl₂. You can make four different isomers, depending on the position of the chlorine atoms.
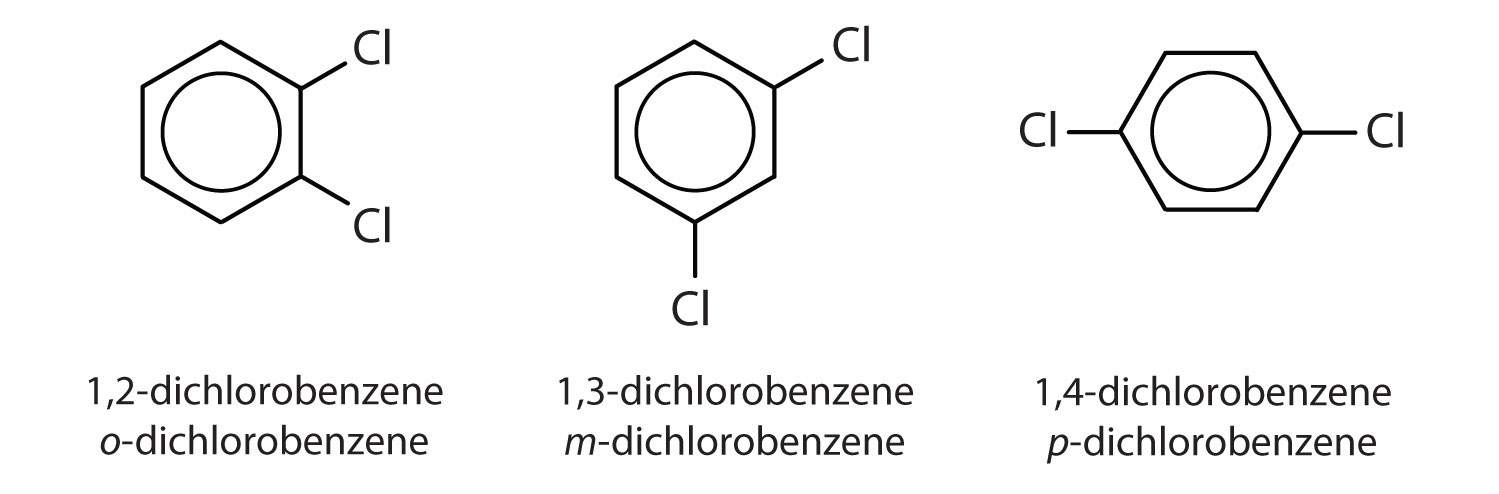
In one case, the Cl atoms are on adjacent C atoms. In the second case, there is a C atom between the ones bearing the Cl atoms. In the third case, the Cl atoms are across the ring from each other.
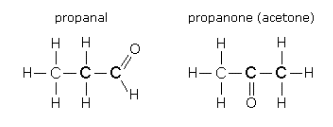
3. Functional Group Isomers
In functional group isomers, the atoms are arranged to make different functional groups.
For example, a molecular formula C₃H₆O could be propanal (an aldehyde) or propanone (a ketone).