Question #38fc0
1 Answer
There are several steps that help us find the lewis structure of iodine pentafluoride.
1)Count the valence electrons.
7+35=42 total valence electrons
2)Form the skeletal structure and add only a single bond (2 electrons) between the central and non central atoms. Then subtract the # of electrons used in this step from the total number of valence electrons.
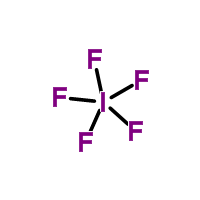
Knowing there are 2 electrons per bond, we subtract 10 from our 42 total valence electrons.
3) Complete the octet of the noncentral atoms. If any electrons remain place them in pairs around the central atom. Subtract the number of electrons used from remaining valence electrons.
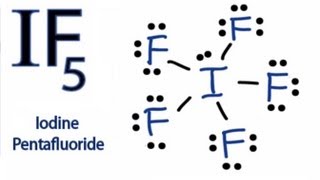
We used 6 electrons on each fluorine so
and
We placed these remaining 2 electrons on the central atom giving us our correct Lewis structure.
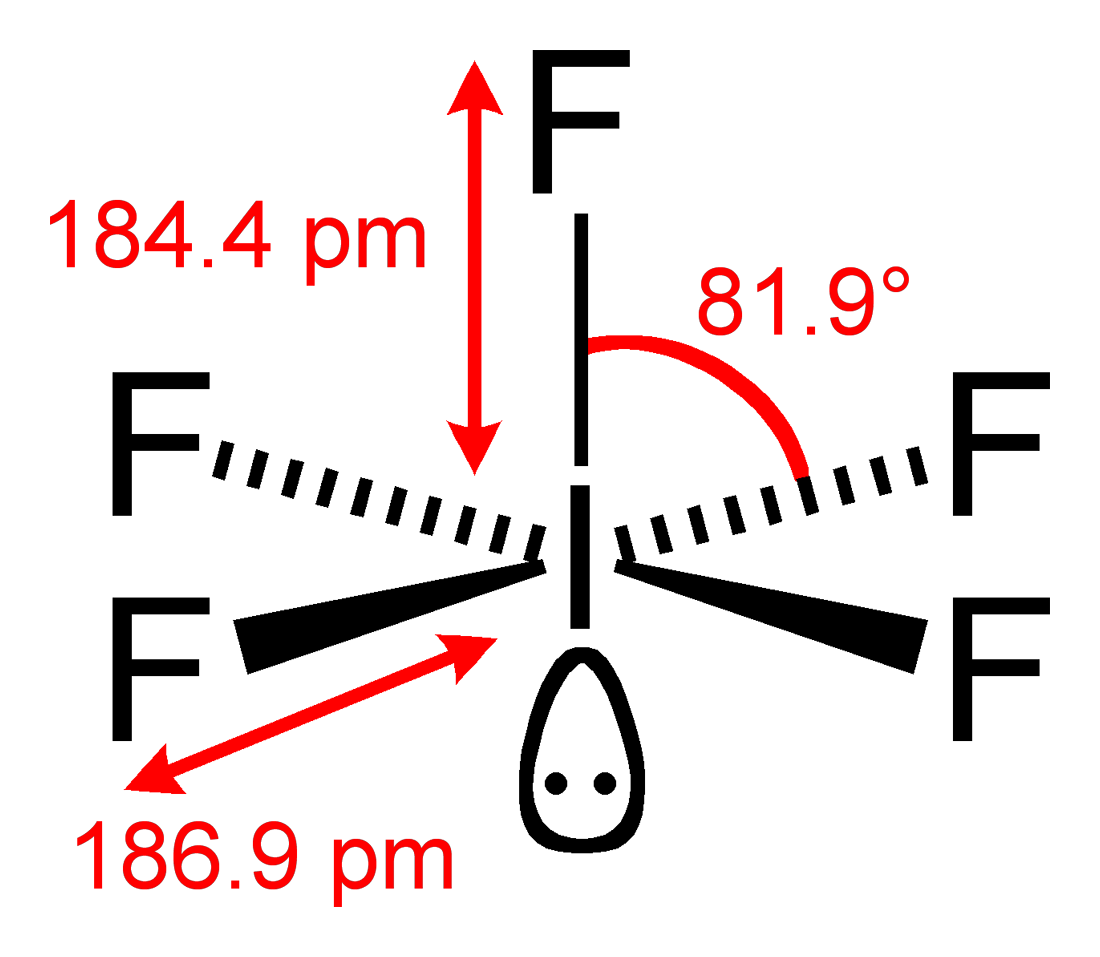
4) Count Regions of High Electron Density (RHED) to obtain VSEPR structure.
There are 6 RHED which give us an octahedral structure according to VSEPR theory.