How many equivalent hydrogens does ethyl acetate have?
1 Answer
Ethyl acetate has three sets of equivalent hydrogens.
Explanation:
The structure of ethyl acetate is

The molecule contains eight H atoms. Some of them are different from each other.
For example, the three H atoms on the left are attached to a carbon bonded to a C=O group.
They are different from the hydrogens on the CH₂ group, which is bonded to an O atom.
And the three H atoms on the right are different from the other two groups.
One way to check whether certain H atoms are equivalent is to replace each H atom with a group like Cl and see if you generate the same compound.
Replacing any H atom on the left hand C gives ClCH₂COOCH₂CH₃.
Replacing any H atom on the CH₂ group gives CH₃COOCHClCH₃.
Replacing any H atom on the right hand C gives CH₃COOCH₂CH₂Cl.
These are three different compounds, so there are three sets of equivalent H atoms.
The NMR spectrum for ethyl acetate confirms this prediction.
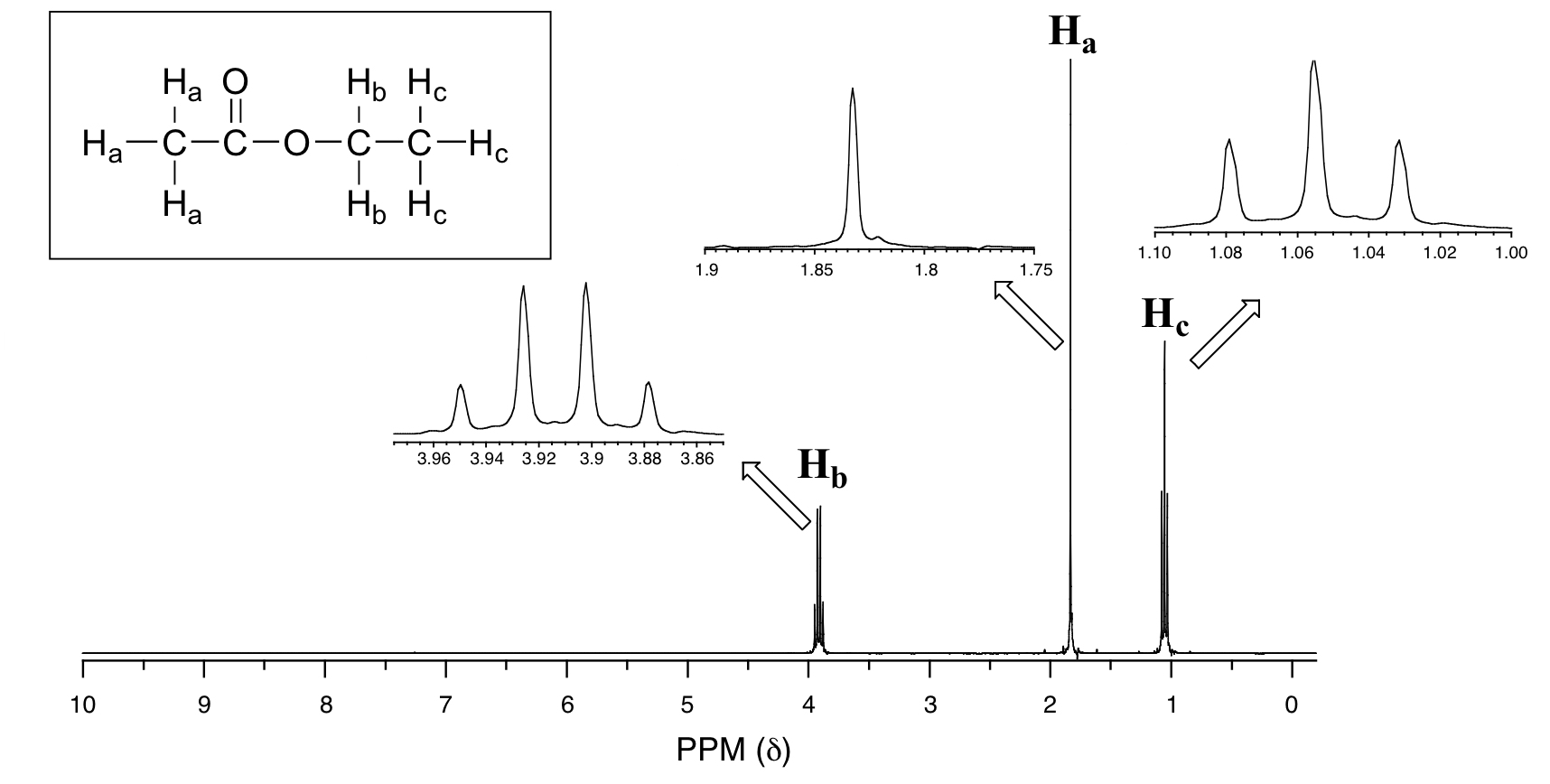
The signal at 1.83 ppm corresponds to the
The signal at 1.05 ppm corresponds to the
The signal at 3.91 ppm corresponds to the

