Why does a lake freeze at the top first, rather than throughout or at the bottom?
1 Answer
This is due to the fact that when water freezes its VOLUME increases.
You may have seen this when someone forgets a bottle of water in a freezer....the water inside the bottle freezes, increases its volume and breaks the bottle.
Now if solid water has a bigger volume it means that has a smaller DENSITY compared, for example, to liquid water.
Density is a measure of how much stuff (mass) you have in a certain volume (
so, if volume increases...DENSITY decreases!!!!
The density of liquid water is
And now the interesting thing...consider 1 kg of iron.
Make it a cube of solid iron...it has a lot of mass packed into a little cube (=high density).
Now take the same amount of iron and build a hollow ball (like a buoy) so that now your mass is "dispersed" into a big volume (=low density).
What happens if you put them in water? The little cube sinks (the water around it cannot support it) the buoy floats because is less dense than the surrounding water!!!
Our idea is that less dense stuff floats on denser one.
It is the same with ice; it is less dense than the surrounding water so it stays afloat (like an iceberg or like oil on water).
The incredible thing is that water (
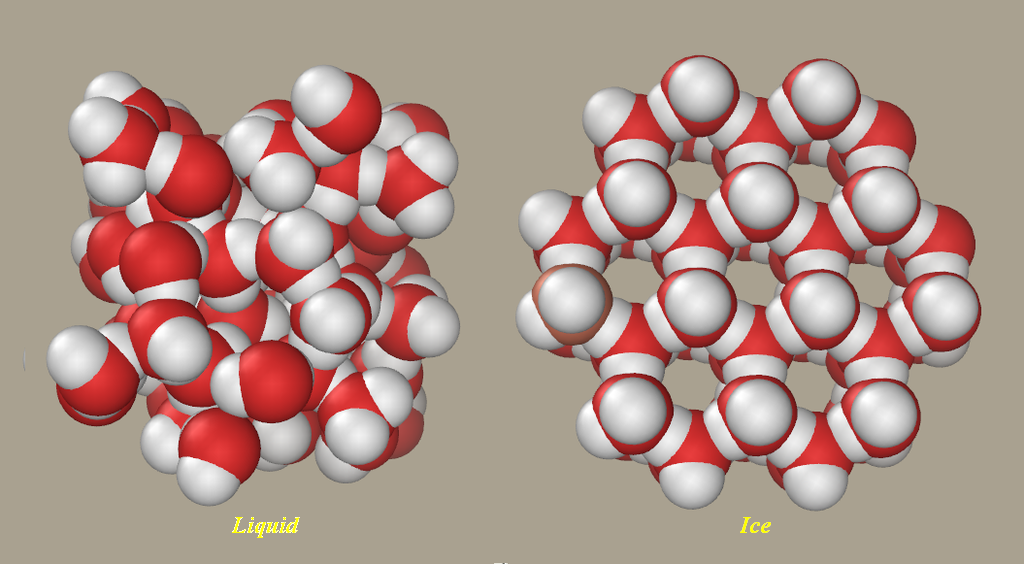
(Picture source: Wikipedia)
You may want to check some theory on:
Floating and density: Stevin's Theorem, Archimedes Principle;
Water and Freezing: the Hydrogen Bond.

