Why do cyclic compounds most commonly found in nature contain six-membered rings?
1 Answer
The cyclic compounds most commonly found in nature contain six-membered rings because they are the best compromise between ring strain and the chances of ring formation.
The ends of a chain of atoms must come together to form a ring.
The ends of 3- carbon and 4-carbon chains are already close together. But joining them to form rings introduces large ring strain and torsional strain.
Cyclopropane and cyclobutane rings are not common in nature.
There is free rotation about the C-C single bonds. As the chains get longer, the chances of the ends getting close enough to form a ring become smaller.
A five-membered ring has little ring strain, but there is torsional strain.

It can get rid of some of the torsional strain by puckering into an envelope shape.
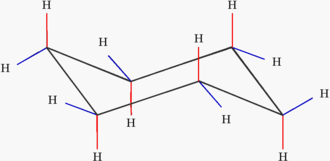
A cyclohexane ring can pucker into a stable chair form that has no ring strain and all the C-H bonds are staggered. This is the most stable arrangement.
Larger rings like cycloheptane and cyclooctane are strain-free, but the chances of the ends of 7- and 8-carbon chains coming close enough to join are small.
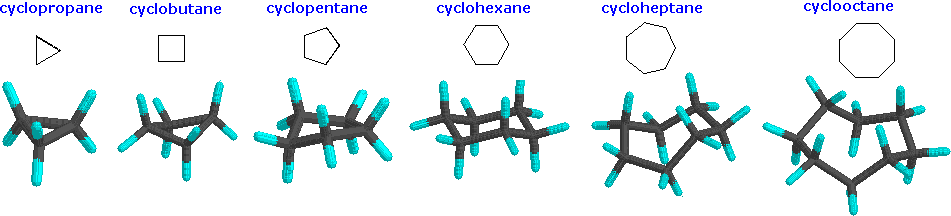
Rings of many sizes occur in nature, but 6-membered rings are the most common.

