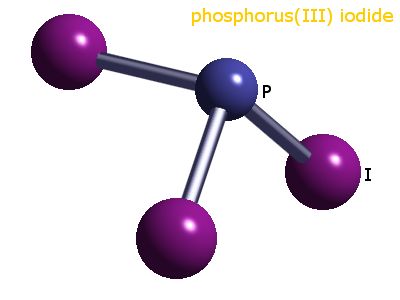
Based on the VSEPR theory, what is the molecular geometry of a molecule of PI3?
1 Answer
Dec 28, 2014
VSEPR Theory allows for the prediction of molecular shapes based on the number of electron pairs that surround their respective atoms.
In order to determine the molecular shape of the
SInce
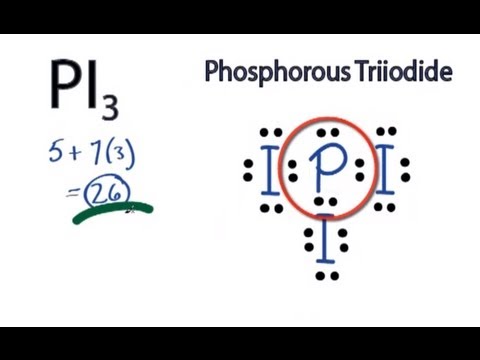
Notice that
Here's a representation of