What are the factors that influence the intensity of an IR absorption band?
1 Answer
The most important factor that influences the intensity of an IR absorption band is the change in dipole moment that occurs during a vibration.
For example, an aldehyde C=O stretch usually occurs near 1730 cm⁻¹. An alkene C=C stretch usually occurs near 1650 cm⁻¹.
The C=O stretch is much more intense than the C=C stretch. The C=O bond is highly polar, so its dipole moment changes considerably as the bond stretches (
Butyraldehyde has an intense C=O absorption at 1731 cm⁻¹.

In contrast, the C=C absorption in 2,3-dimethylbut-2-ene is not detectable.

The C=C bond is nonpolar, and the molecule is symmetrical, so there is no change in dipole moment during the stretch.
You can see the C=C stretch in a terminal alkyne such as oct-1-ene.
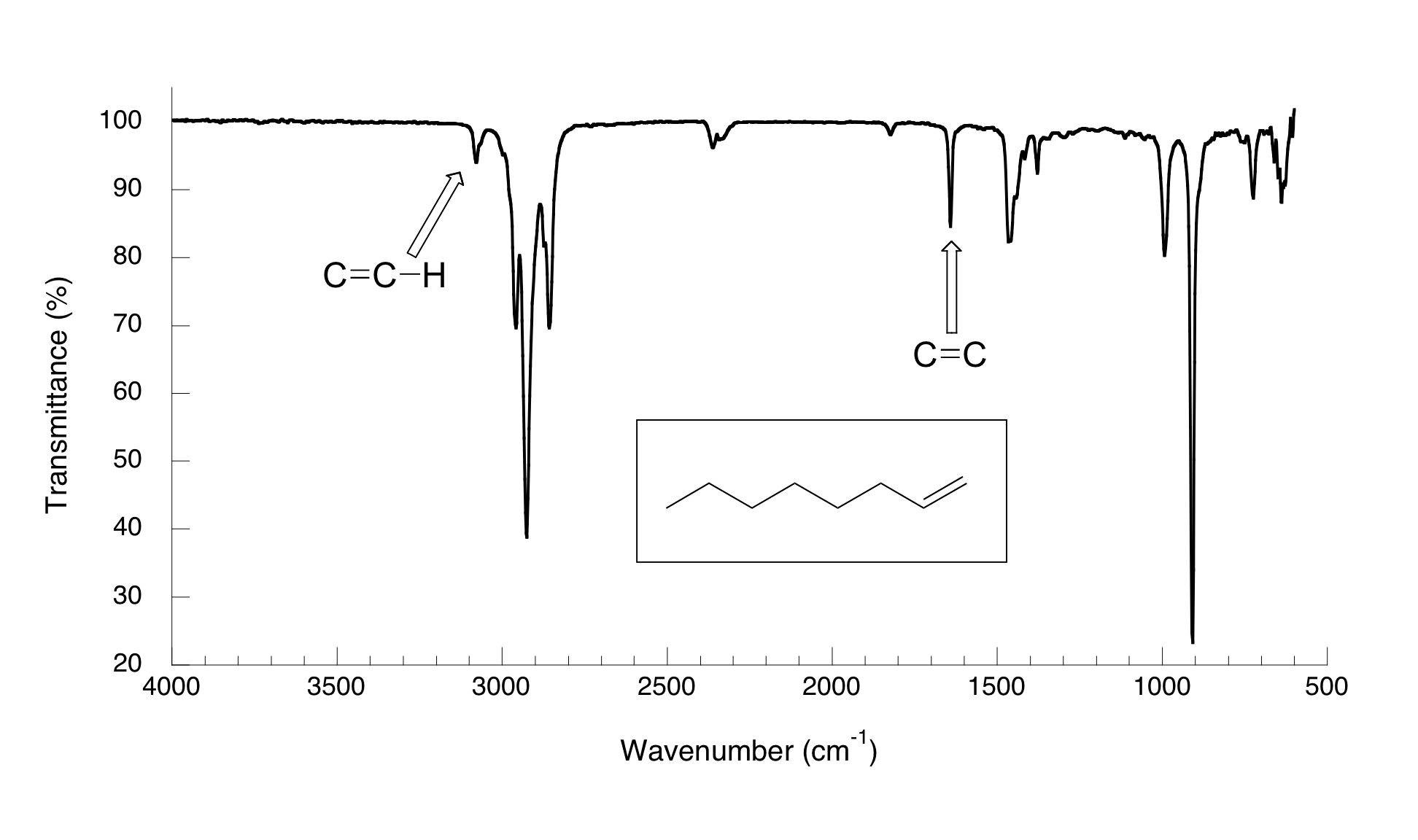
The bond is not completely symmetric, so there is a small change in dipole moment. But the C=C peak at 1644 cm⁻¹ is still quite weak.

