Can carbon form 4 bonds?
1 Answer
A carbon atom can and does form four bonds, which is among the reasons why orbital hybridization proved to be such a successful theory.
Neutral carbon is located in group 14, period 2 of the periodic table and has an atomic number of 6 and, subsequently, a total of 6 electrons.
Out of these six electrons it has, four are its valence electrons, i. e the electrons located in the outermost shell of the atom. Carbon's electron configuration looks like this
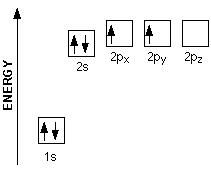
Notice that out of the 4 valence electrons a carbon atom has, only 2 are unpaired and thus available for bonding, the ones located in the
Here's where orbital hybridization came into play. According to this theory, when the carbon atom is in an excited state, one of the two electrons located in the
Then the
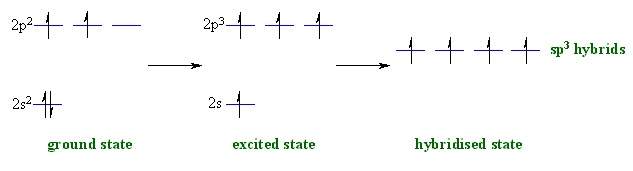
As a result, carbon now has 4 unpaired valence electrons with which it can form four bonds.

