Question #69999
1 Answer
The calculation makes several assumptions, but I think you need 0.75 mL of 25 % ammonia solution.
Step 1
HPMCP is hydroxypropylmethylcellulose phthalate.
The repeating unit in HPMCP is
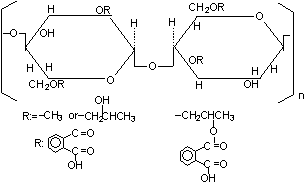
This gives a formula of C₃₅H₄₁O₁₈ and a molar mass of 749.7 g.
HPMCP is a diprotic acid. Let's write it as H₂A.
Then
H₂A + 2NH₃ → 2NH₄⁺ + 2A⁻
The density of 25 % aqueous NH₃ is 0.911 g/cm³.
Step 2
HPMCAS is hydroxypropylmethylcellulose acetate succinate. The repeating unit in HPMCAS is

This gives a formula of C₃₂H₅₀O₂₀ and a molar mass of 754.7 g.
HPMCAS is also a diprotic acid. Let's write it as H₂A.
Then
H₂A + 2NH₃ → 2NH₄⁺ + 2A⁻
Step 3
Total volume = 0.70 mL + 0.050 mL = 0.75 mL


