Question #4c3aa
1 Answer
For neutral atoms, you'll always have a net charge equal to zero. This happens because a neutral atom has equal numbers of protons and electrons.
Elements are listed in the periodic table in order of their atomic numbers, symbol
The number of neutrons an atom has in its nucleus is determined by using its mass number, symbol
which is equivalent to
So, for any element in the periodic table, you'll have
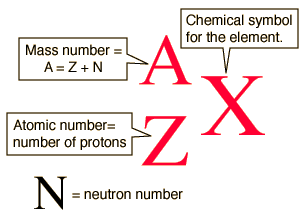
Net charge is determined by comparing the number of protons and the number of electrons an atom has. So,
I'll redirect you to a more detailed answer on determining net charge because I don't want this answer to become very long:
http://socratic.org/questions/i-want-to-know-how-to-get-the-net-charge-for-each-element

