Question #95d51
1 Answer
Simply put, sacrificial protection is a method used to prevent corrosion. This is done by connecting the metal we want to protect from corrosion, usually iron or steel, to a more electrochemically reactive metal - see metal activity series.
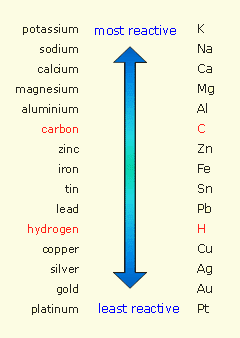
The more reactive metal lose electrons more easily and donates them to the less reactive metal, replacing the electrons it lost through oxidation. In essence, the more reactive metal becomes a sacrifical anode and the protected metal becomes the cathode, which does not corrode - the same principle behind galvanic cells.
Usually zinc or magnesium are used to protect iron from corrosion. The oxidized iron will be reduced back to its original state by the electrons coming from zinc or magnesium, basically reversing the oxidation process.
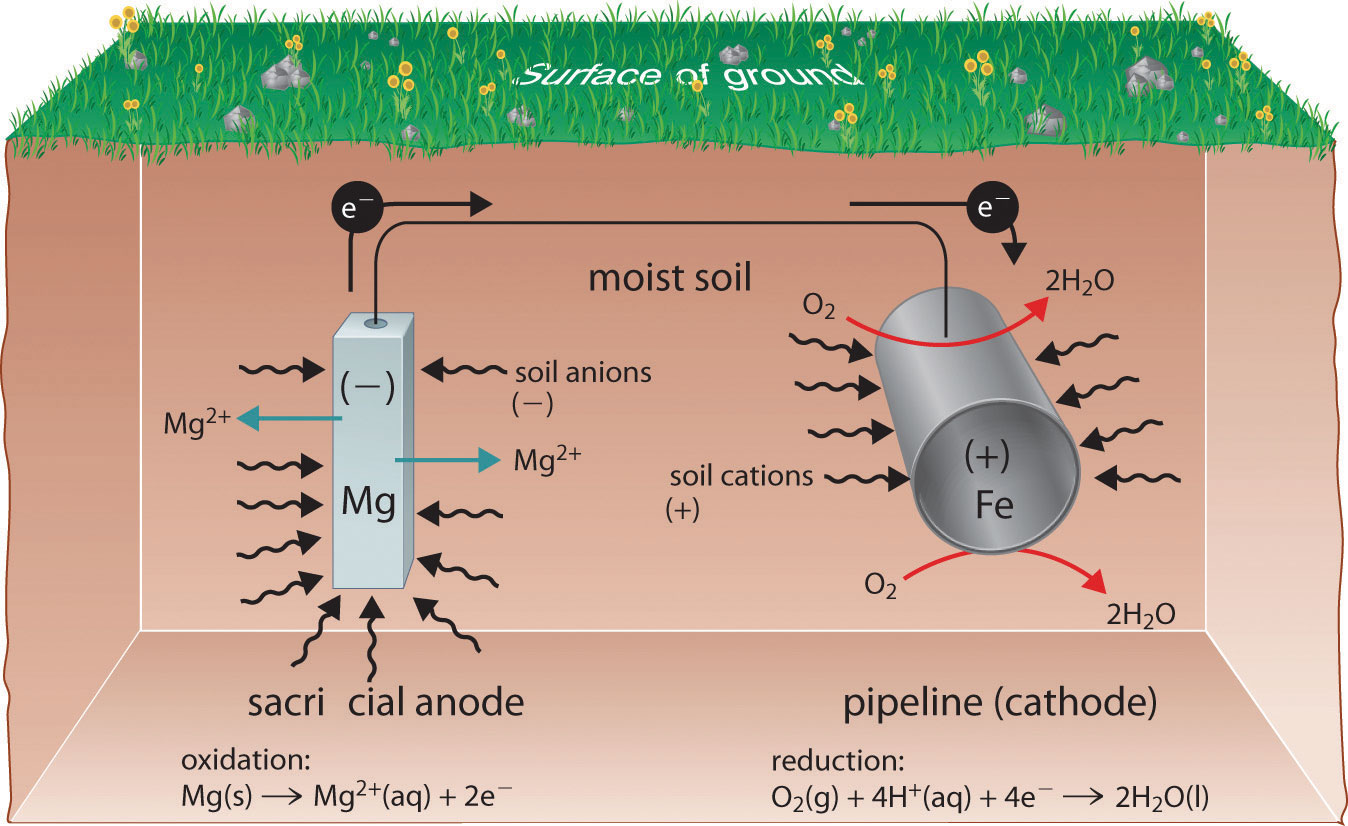
Sacrificial protection has many applications, as you would imagine, most commonly being used to protect
- Above-ground and undergound tanks;
- Pipelines;
- Ship hulls;
- Oil rigs;
Here's a very nice animation showing how sacrificial protection works

