Question #2729e
2 Answers
Only Na is electrically neutral.
An electrically neutral species has no charge.
Na⁺, K⁺, and Ca²⁺ all have positive charges.
Cl⁻ has a negative charge.
Na is the only species with no charge.
It is therefore electrically neutral.
A neutral element must have the number of protons it has in its nucleus equal to the number of electrons that surround its nucleus. When this happens, the atom is said to have no net charge.
If an atom has too many protons than electrons, it will carry a positive charge that reflects this difference. In your case, notice that you have
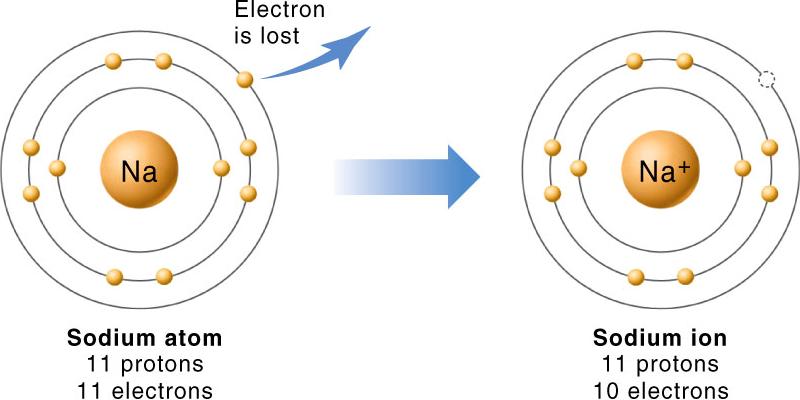
In sodium's case, neutral sodium has 11 electrons and 11 protons and the symbol
Likewise, if an atom has too many electrons, it will carry a negative charge. In your case,
So, if you look at an atom and it has no positive or negative charge attached, i.e. +1, +2, -1, -2, and so on, it means it's a neutral atom.
Basic principles of atoms and ions to help the student answer the particular question with additional info here http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch2/atom_ion.html



