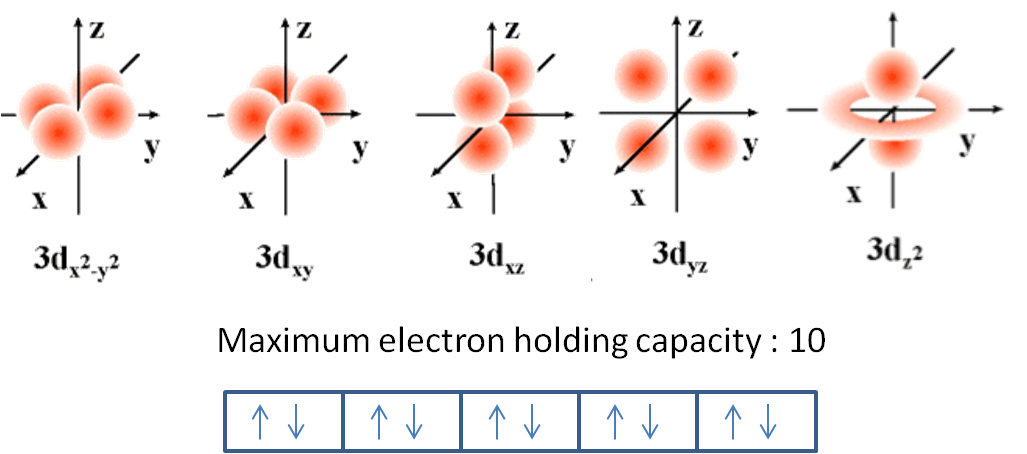
The maximum number of electrons allowed in an individual d orbital is?
1 Answer
Apr 4, 2015
An individual d-orbital can hold no more than 2 opposite-spin electrons.
Individual orbitals can hold no more than 2 electrons each, regardless of the type of orbital you've got.
The d-subshell is comprised of 5 d-orbitals, which means it can hold a maximum of 10 electrons, 2 from each d-orbital.