Which of the following is an incorrect designation for an atomic orbital? a) 1s b) 4f c) 3s d) 2d e) 2p
1 Answer
Apr 4, 2015
The answer is d) 2d.
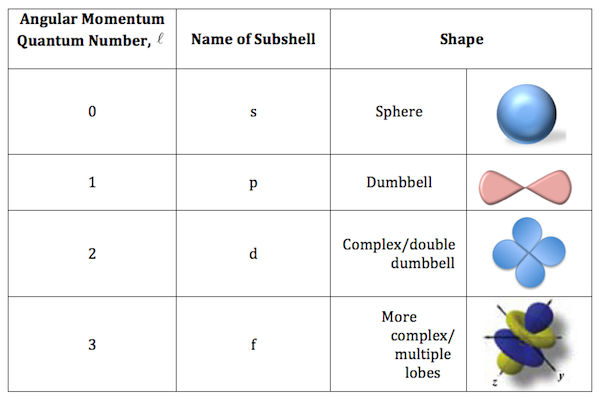
Without going into too much detail, the 2d orbitals cannot exist because they are not allowed solutions to the Schrodinger equation.
Simply put, the second energy shell, designated by a principal quantum number equal to 2, or
For the second energy level, the allowed values for the angular momentum quantum number, or
D-orbitals are allowed starting with the third energy level, or