How many straight-chain isomers are there of hexene, #C_6H_12#?
2 Answers
Five isomers
The double bond may be between carbon 1 and 2, or 2 and 3, or 3 and 4. If the double bond is between carbon 2 and 3 or 3 and 4 there are 2 geometric isomers for each case (cis- and trans)
There are 5
Explanation:
Of course, there are no straight chain answers, because most bonds rotate around the
These bonds can be between
(The others are mirror-images if you count from the other side of the chain.)
Now, with every double bond, there are two possibilities: cis or trans
If the rest of the molecule is on the same side it's called cis:
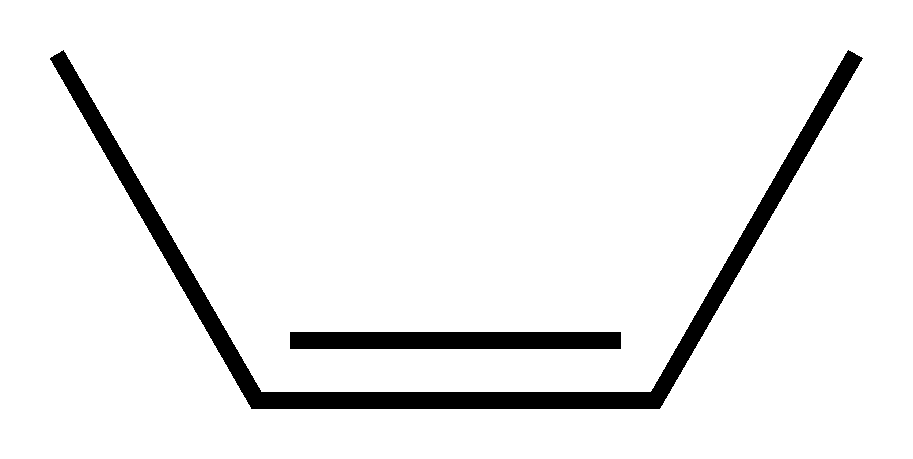
if not then it's called trans:
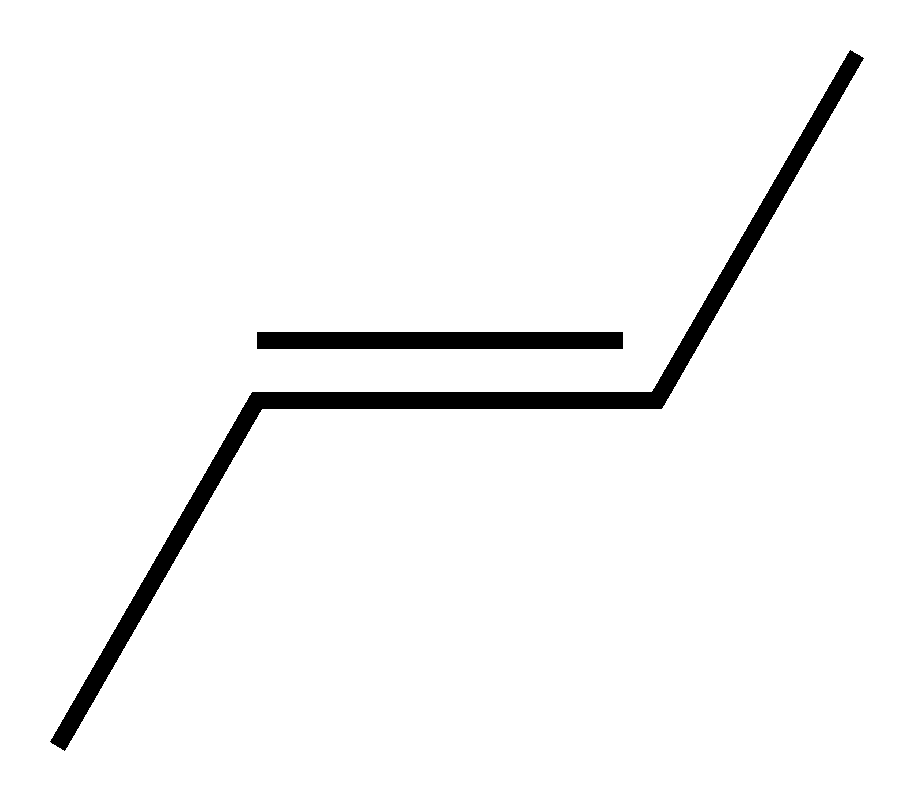
For the double bond between
Another way of showing if a molecule is cis or trans is using *E-Z notation*.
(Pictures from Wiki of the two possibilities of but-2-ene)



