Question #e37e4
1 Answer
May 11, 2015
The difference in electronegativity between silicon,
According to the Pauling electronegativity scale, silicon has an electronegativity value of 1.90. Fluorine, which is the most electronegative atom, has an electronegativity value of 3.98.
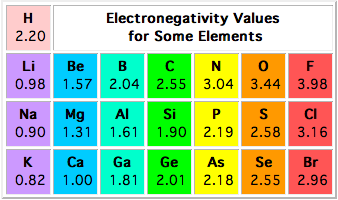
As a result, the difference between the two will be

