Question #f563c
1 Answer
May 13, 2015
Zinc reacts with hydrochloric acid to produce hydrogen gas because it's more reactive than hydrogen, and thus displaces the latter from an acid.
By comparison, copper cannot displace hydrogen from
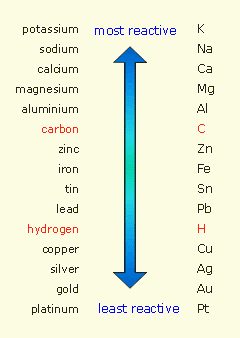
So, zinc will react with hydrochloric acid to produce zinc chloride and hydrogen gas
Zinc will reduce the hydrogen and get oxidized in the process.
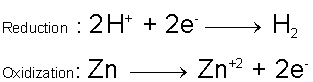
In copper's case, no reaction takes place when you place it in hydrochloric acid
The video shows an experiment to determine the placement of three different metals (Cu, Zn and Mg) on the activity series.
Video from: Noel Pauller


