Question #6f186
1 Answer
May 13, 2015
This is another example of a reaction in which the metal cannot displace hydrogen from the acid.
Take a look at the metal activity series
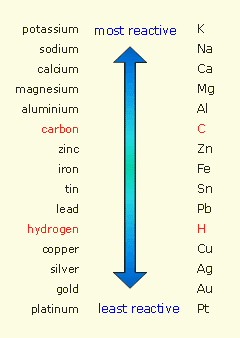
Any metal located below hydrogen in the table will not be able to displace it from acids. By comparison, any metal located above hydrogen in the table will be able to displace hydrogen from acids.
Once again, the single replacement reaction does not take place
Silver's placement on the activity series is very close to copper - another metal which will not react with HCl. The video shows an experiment to determine the placement of three different metals (Cu, Zn and Mg) on the activity series.
Video from: Noel Pauller
Hope this helps!


