What is the difference in electronegativity between magnesium and chlorine when they react to form magnesium chloride?
1 Answer
May 24, 2015
The difference in electronegativity between magnesium and chloride is equal to 1.85.
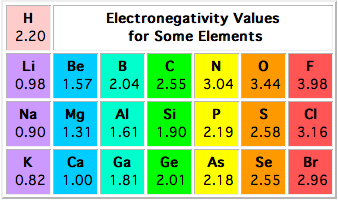
As you can see, the electronegativity values of the two elements are 1.31 for magnesium and 3.16 for chlorine. You can determine the difference in electronegativity between the two by subtracting the smaller value from the bigger one
This difference in electronegativity values implies that the bond between the magnesium cation,

