The liquefied hydrogen halides have the normal boiling points given above. The relatively high boiling point of HF can be correctly explained by which of the following?
1 Answer
The answer is (e) hydrofluoric acid molecules tend to form hydrogen bonds.
Here's a diagram showing the boiling points of the hydrogen halides
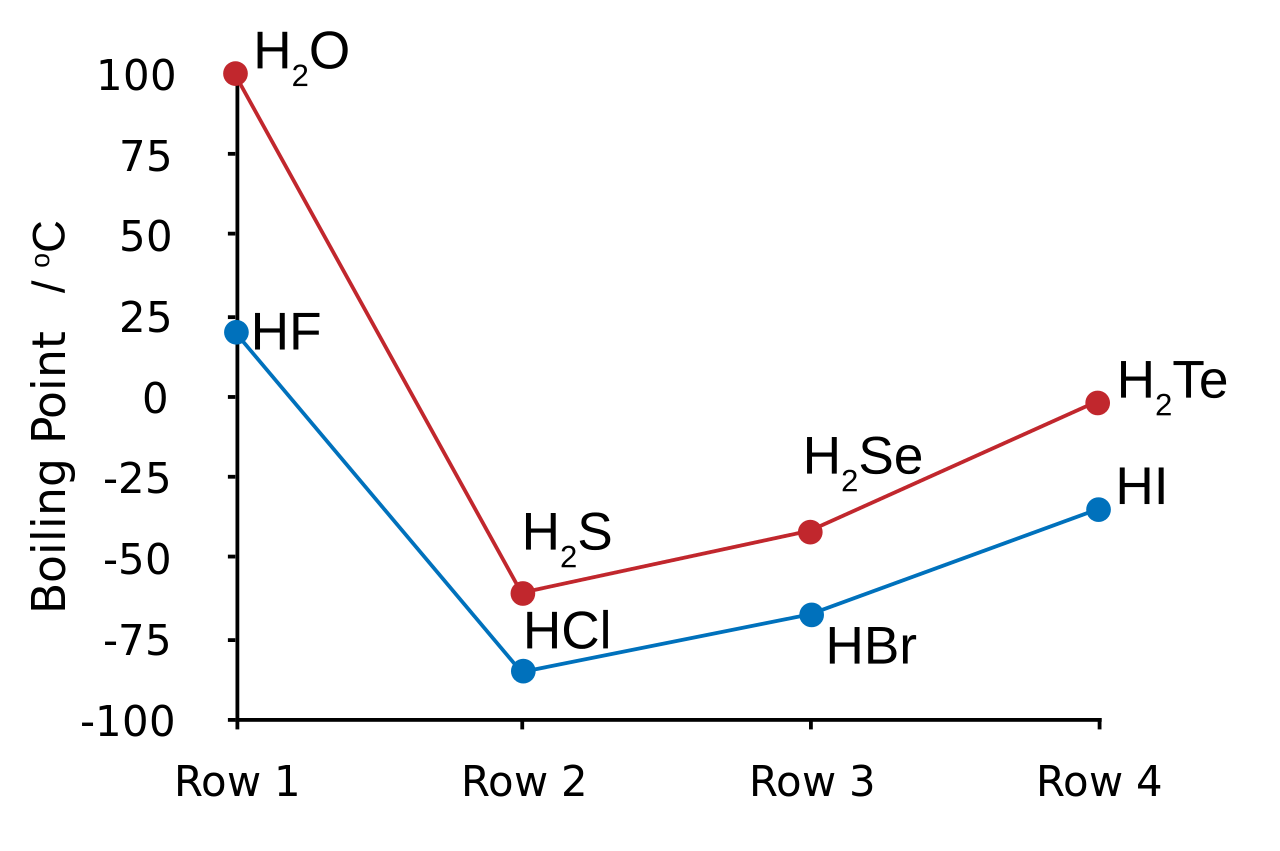
Hydrogen fluoride's boiling boint is explained by the fact that its molecules exhibit the strongest intermolecular forces out of all the hydrogen halides.
That is the case because hydrogen fluoride can form hydrogen bonds, which are a special case of dipole-dipole interactions that occurs when hydrogen is bonded to fluorine, nitrogen, or oxygen.
Fluorine's very high electronegativity will cause a partial positive charge to develop on the hydrogen atom. At that point, one of the lone pairs of electrons located on another fluorine atom will be attracted to that partial positive charge.
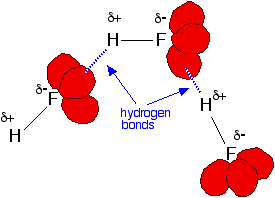
As a result, a hydrogen bond is formed. Hydrogen bonds are particularly strong intermolecular forces, which is why hydfrogen fluoride's boiling point is so high compared with the boling points of the other hydrogen halides.
You can read more on hydrogen halides and hydrogen bonding here:

