Question #aacd0
1 Answer
The answer is d) solvated electrons.
Explanation:
When sodium atoms dissolve in liquid ammonia, they lose an electron to become sodium cations,
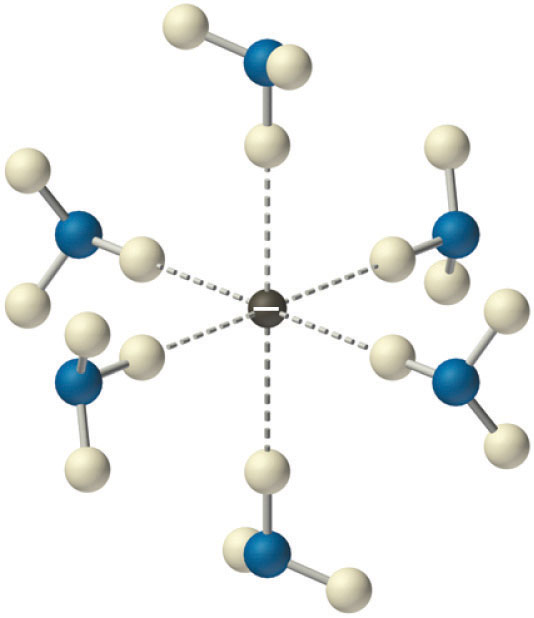
Being negatively charged, the electrons will attract the partial positive hydrogen atoms of the ammonia molecules. Likewise, the sodium cations will attract the lone pair that's on the nitrogen atom.
The attraction between a solvated electron and the hydrogen atoms present on the ammonia molecules is very weak, so, for all intended purposes, these electrons are free to move in solution.
The availability of these electrons is what makes solutions of sodium dissolved in ammonia are very strong reducing agents.
So, the presence of solvated electrons makes a solution of sodium dissolved in ammonia a very strong reducing agent.

