Hello. Can someone help me with drawing hydrogen bonds among different molecules, with an example below? I understood how water molecules are linked to each other by hydrogen bonds. But I have a problem drawing these bonds in other biomolecules. Thanks!
1 Answer
Here's what you must look for in order to be able to draw hydrogen bonds.
Explanation:
As you know, hydrogen bonds are a special case of dipole-dipole interactions in which you have a hydrogen atom bonded to one of the three most electronegative atoms in the periodic table: fluorine, oxygen, or nitrogen.
In essence, a hydrogen bond is formed when the partial positive charge that appears on the hydrogen atom of one molecule is attracted to a lone pair of electrons located on another molecule.
This is why each water molecule can form hydrogen bonds to 4 other water molecules.
So, if you are looking to draw a hydrogen bond between two molecules, look for two things
- hydrogen atoms attached to fluorine, oxygen, or nitrogen on one molecule;
- lone pairs of electrons on another molecule.
Here are some examples of how hydrogen bonds are formed between various molecules and water
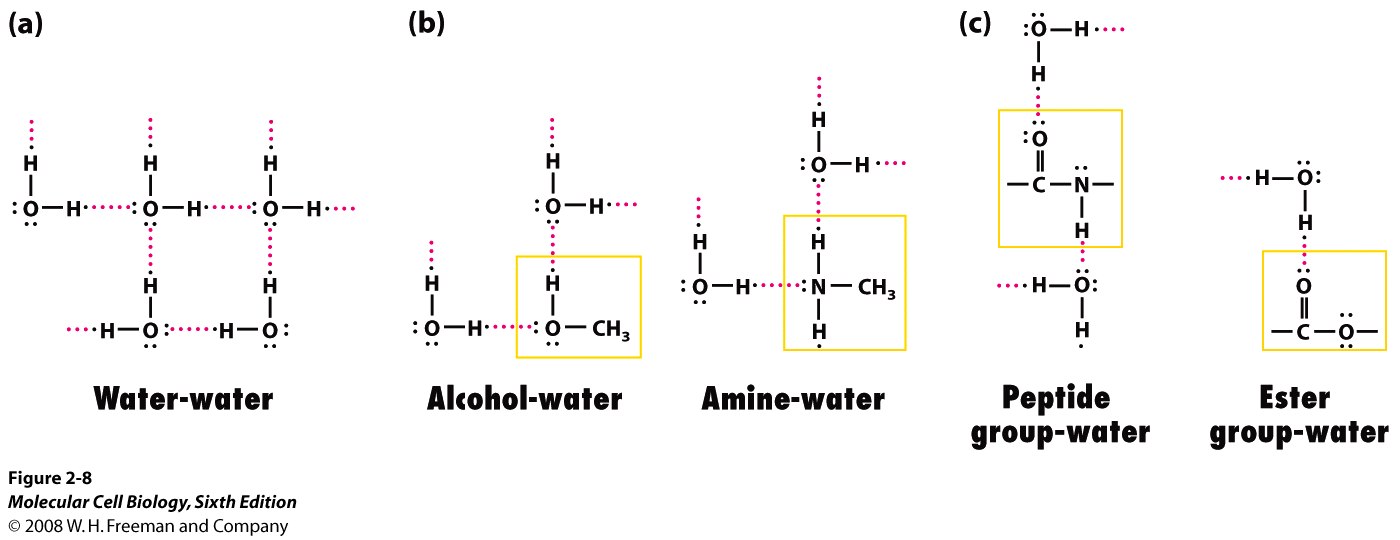
Here are how hydrogen bonds are formed between side chains in proteins (left side of the image)
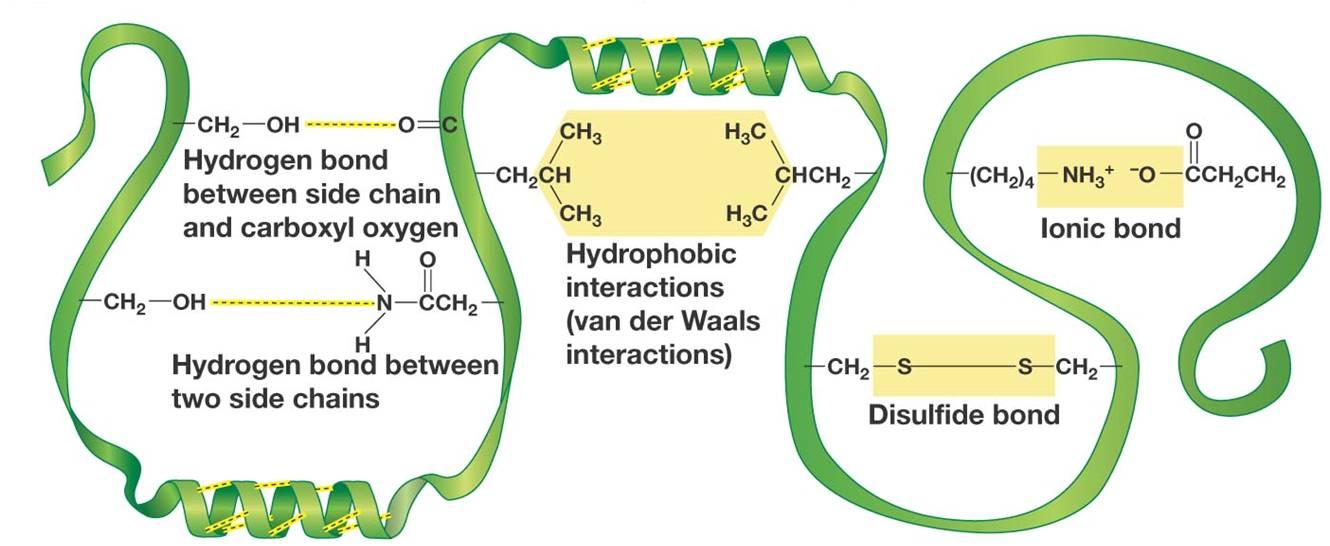
The hydrogen bond is formed between the partial positive hydrogen atom of the side chain and a lone pair of electrons present on the oxygen atom of carboxyl group.
Likewise, a similar partial positive hydrogen can form a hydrogen bond with the lone pair of electrons present on the nitrogen atom of an amine.
So, as a conclusion, always look for partial positive hydrogen atoms (bonded to fluorine, oxygen, or nitrogen) and lone pairs of electrons, and draw hydrogen bonds using dotted lines,


