Question #dcae6
1 Answer
Yes, hydrogen bonding can (and will) take place in that case.
Explanation:
FULL QUESTION
For Question Number 1 Part B. Can the alcohol group (OH) interact to a Lone oxygen atom
Please explain why part B is hydrogen bonding interaction.
As you know, hydrogen bonding is a special case of the more general dipole-dipole interactions characterized by a covalent bonding that takes place between a hydrogen atom and three of the most electronegative atoms in the periodic table, oxygen, nitrogen, and fluorine.
This pairing between hydrogen and three of the most electronegative elements gives molecules the possibility of forming hydrogen bonds.
However, molecules that do not contain such a covalent bond can still act as either hydrogen bond acceptors or hydrogen bond donors.
The interaction between the negative charge located on the phosphate group and the positive charge located on
However, the electrostatic attraction between the negative charge present on the oxygen in the side-chain of
This happens of course because you don't have a full positive charge on the hydrogen atom.
However, a hydrogen bond will most definitely form.
A lone pair of electrons present on the oxygen atom in the
The oxygen atom acts as a hydrogen bond acceptor, and the
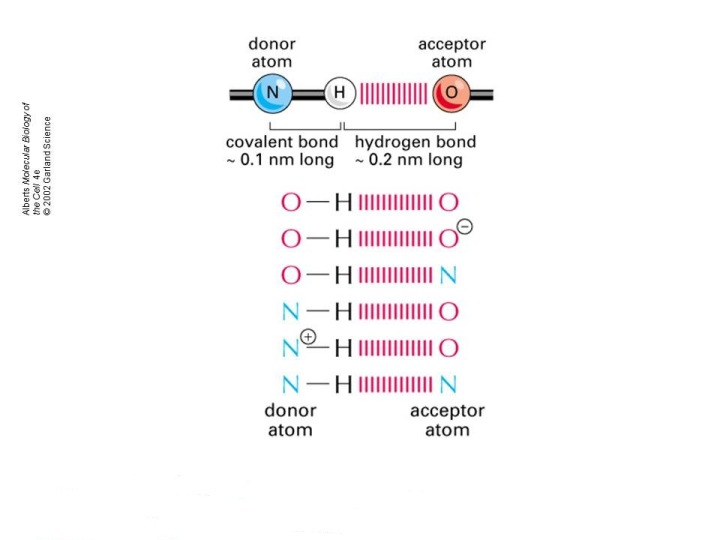
Because it lacks a bond to a hydrogen atom, the oxygen belonging to the side-chain of
This is not the case for the side-chain of
Read more about hydrogen bond donors and acceptors here:

