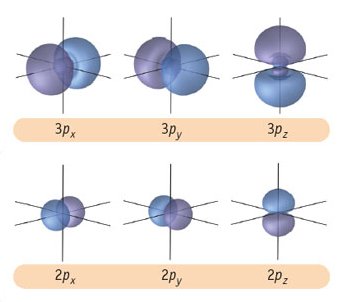
How are s orbitals different from p orbitals?
1 Answer
Jul 25, 2015
The s orbital is spherical, while the p orbital is shaped like a dumbbell. Due to these shapes, the s orbital has only one orientation, while the p orbital has three degenerate orientations (
This is why you write
The s orbital has nodes that lay within its spherical boundaries at select radial distances
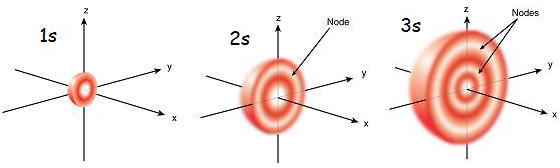
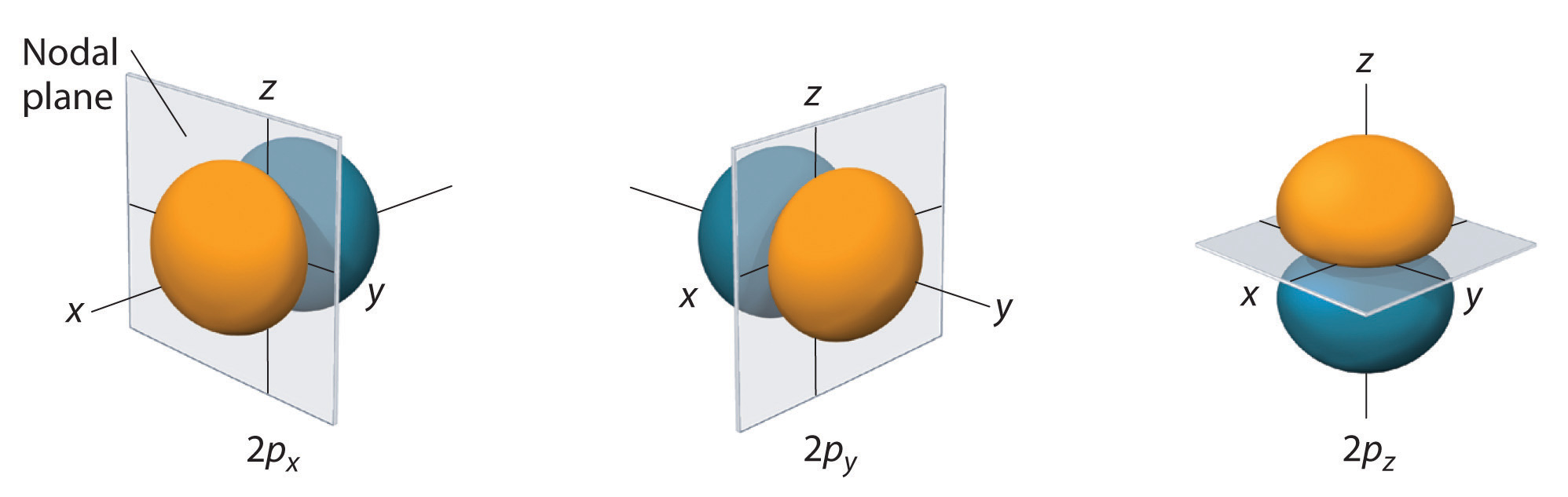
Whereas the s orbital only increases in size and varies due to the quantum number