Question #67733
1 Answer
Actually, chlorine does not have a net charge in calcium hypochlorite.
Explanation:
Calcium hypochlorite, or
The calcium cation has a ner charge of
Now, because the chlorite anion is actually a covalent compound, the chlorine and the two oxygen atoms that make up this ion do not have net charges.
However, they do have formal charges, which are charges that are assigned to atoms that a part of a molecule.
You can assign formal charges by considering that the bonding electrons that two atoms have in a molecule are shared equally between those atoms, irrespective of electronegativity values.
Now, without going into too much detail about this, let's say that the Lewis structure of the chlorite anion looks like this (the ion has other resonance structures as well, but I'll ignore them for simplicity)
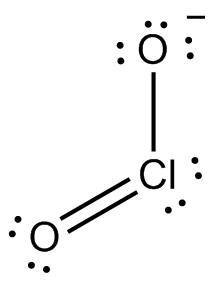
To assign a formal charge to the chlorine atom, you need to compare the number of valence electrons a neutral chlorine atom has with the number of electrons it gets in the molecule, provided like I said that bonding electrons are shared equally.
So, a neutral chlorine atom has 7 valence electrons. In this structure, chlorine gets 4 electrons from the two lone pairs and 1 electron from each bond it forms with the oxygen atoms.
This means that it has a total of
Since this is equal to the number of valence electrons a neutral chlorine atom has, its formal charge will be zero.
So, as a conclusion, atoms that are a part of a covalent compound, i.e. a molecule, do not have net charges, they have formal charges.

