How do enzymes affect rate of reactions?
1 Answer
Enzymes will increase the rate of a chemical reaction by reducing the activation energy needed to make the reaction get started.
Explanation:
Enzymes are protein molecules which serve as catalysts for chemical reactions.
A catalyst is a substance which will decrease the activation energy for a reaction.
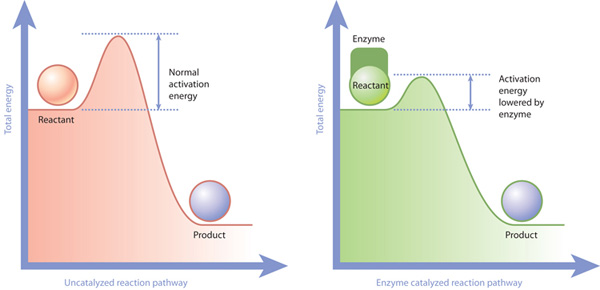
So why is this important? Enzymes will make reactions occur much more easily, quickly and more efficiently than they ever would without the enzyme.
Here is a video of an enzyme demonstration I like to share with my students. The video shows how an enzyme present in our saliva (spit) can aid in the process of digestion by acting on starches present in the foods we eat.
Make sure to pay attention to how quickly the starch changes with and without the added enzyme!
video from: Noel Pauller
This video shows catalase enzyme (from liver) being used to aide the reaction which breaks down hydrogen peroxide.
Hope this was helpful!

