Question #e6518
1 Answer
Explanation:
There is more than one type of electronegativity, each being calculated using different methods, but the most common is the Pauling electronegativity scale.
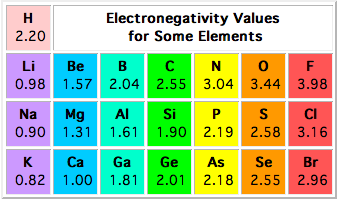
According to this scale, hydrogen, which is located in group 1, period 1 of the periodic table, has an electronegativity value of
Alternatively, you can try to calculate hydrogen's Mulliken electronegativity by using the Mulliken equation, which uses electron affinity and ionization energy values to estimate the electronegativity of an element.
In hydrogen's case, you have
#E_(ea) = "72.77 kJ/mol"" "# and#" "E_i = "1312 kJ/mol"#
The Mulliken equation looks like this
#chi = 1.97 * 10^(-3) * (E_i + E_(ea)) + 0.19#
In this case, you would get
#chi_"hydrogen" = 1.97 * 10^(-3) * (72.77 + 1312)"kJ/mol" + 0.19#
#chi_"hydrogen" = 1.97 * 1384.77 * 10^(-3) + 0.19#
#chi_"hydrogen" = 2.73 + 0.19 = "2.92 kJ/mol"#
Other types of electronegativities include the Allred - Rochow electronegativity, which uses effective nuclear charge, and ALlen electronegativity, which uses the average energy of the valence electrons to find an atom's electronegativity.

