How many orbitals are in each sublevel?
1 Answer
That depends on the subshell.
Explanation:
Electrons that surround an atom's nucleus are distributed on specific energy levels, or shells.
Each shell is made up of a different number of subshells. More specifically, the number of subshells a shell can have increases as you move away from the nucleus.
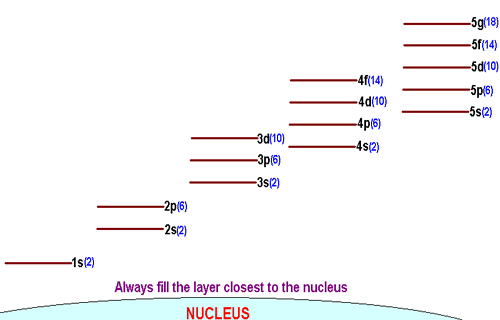
You can use quantum numbers to illustrate this point.
The principal quantum number,
Now, the number of subshells is given by the angular momentum quantum number,
This means that you will have
n = 1 implies l = 0 -> the first shell only has one subshell, sn = 2 implies l = 0, 1 -> the second shell has two subshells: s and pn = 3 implies l = 0, 1, 2 -> the third shell has three subshells, sp, p, and d
vdots
and so on.
The number of orbitals each subshell contains is given by the magnetic quantum number,
So, for example, how many orbitals would you say the 2p-subshell has?
Well, the 2p-subshell has
m_l = {-1, 0, 1} -> the 2p-subshell contains 3 orbitals.
How about the 3d-subshell?
For the 3d-subshell, you know that
m_l = {-2, -1, 0, 1, 2} -> the 3d-subshell contains 5 orbitals.
So, as a conclusion, you get th number of orbitals per subshell from the principal quantum number,
The magnetic quantum number, the ones that tells you exactly how many orbitals you get per subshell, will always take values from

