Question #09e47
1 Answer
A physicist who proved that electrons of an atom are orbiting the nucleus in energy levels (shells) .
Explanation:
Before Niels Bohr, Rutherford said that the electrons are orbiting the nucleus in levels or orbits by using his planetary model of the atom. Rutherford didn't bring out the idea of these levels being actually energy levels. This is his most famous discovery.
After this, Niels Bohr came into play and he used the Hydrogen emission spectrum to explain that the levels electrons are said to be orbiting are actually energy levels. He said that the energy level or shell closest to the nucleus has the lowest energy and gets higher when moving away from it. He explained that using the lines that are in the emission spectrum.
Since he used Hydrogen, he said that the H atoms in the cathode ray tube take energy and goes to higher energy levels when a high voltage is given to the ends of the tube. We say that when the atom is given high enough energy for it to be ionized, the outermost electron goes to energy level
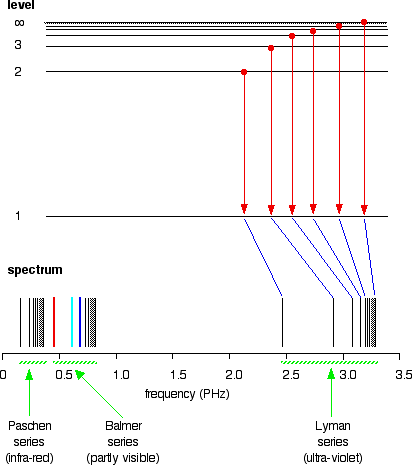
source : http://www.chemguide.co.uk/atoms/properties/hspectrum.html
Since the high energized state is not stable, the excited atoms give out energy packets and fall onto lower energy levels. The H atom has only one electron and there are many ways that this electron can fall onto its ground state which is energy level one. it can go from energy level
That is how Bohr proved that electrons orbit the nucleus in energy levels.
here are some wikipedia pages u can read more info if you want
about niels bohr : https://en.wikipedia.org/wiki/Niels_Bohr
bohr's atomic model https://en.wikipedia.org/wiki/Bohr_model

