What is the maximum number of orbitals in the p sub level?
1 Answer
Oct 31, 2015
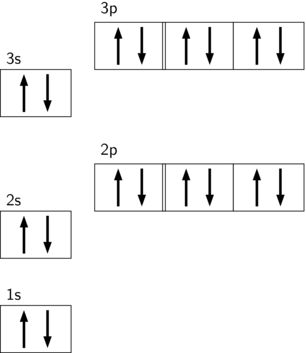
A p sublevel has three orbitals.
Explanation:
P sublevels have three orbitals, each of which can hold a maximum of 2 electrons with opposite spins, for a total of 6 electrons.
The following orbital diagram represents the filling of the orbitals of the noble gas argon. Note that the 2p and 3p sublevels each have three orbitals with six electrons each.
Argon