Question #48cf9
1 Answer
The geometry of
Explanation:
The best way to understand geometry is by drawing the Lewis dot structure of the molecules.
The Lewis dot structure of
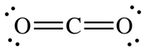
The Lewis dot structure of
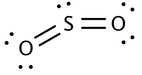
The geometry of
The geometry of
The geometry of
The best way to understand geometry is by drawing the Lewis dot structure of the molecules.
The Lewis dot structure of
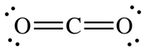
The Lewis dot structure of
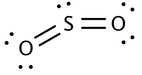
The geometry of
The geometry of