Question #6fe8f
1 Answer
Nov 2, 2015
Sublimation.
Explanation:
The most famous example are moth balls. You can leave it in one corner and after some time, you will see that its volume/radius is slightly diminishing little by little until nothing is left but the strong smell of napthalene.
Sublimation is an endothermic process (meaning, the system absorbs energy, usually heat, from its surroundings) and only occurs when the substances temperature and pressure point reaches below its triple point (see phase diagrams ).
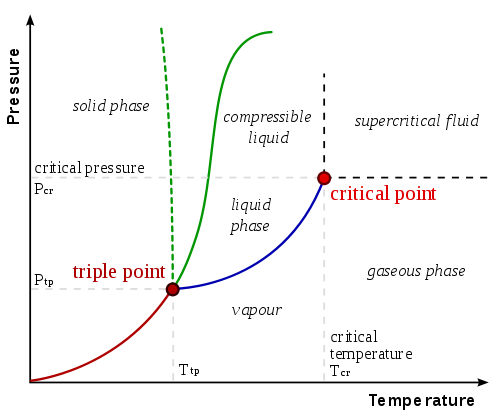
This means that substances that sublime have a specific triple point that should be reached before it can actually undergo this process.

