How many d orbitals must be occupied by single electrons before the electrons begin to pair up?
2 Answers
All of them.
Explanation:
Degenerate obitals, which are orbitals that have the same energy level, must all be occupied with one electron before you can start adding the second electron to each of these orbitals - this is known as Hund's Rule.
So regardless of how many degenerate orbitals you have per subshell, they must all be occupied by a single electron before pairs of electrons can be formed.
In your case, the d-subshell has a total of five orbitals
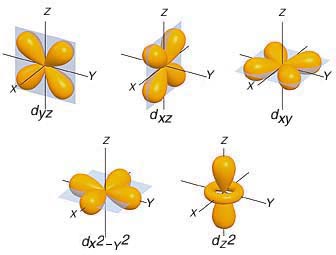
This means that you would start adding one electron of the same spin in this order
#d_(yz) -> d_(xz) -> d_(xy) -> d_(x^2 - y^2) -> d_(z^2)#
After all these orbitals get occupied by one electron of the same spin, you can start adding different spin electrons in the same order
#d_(yz) -> d_(xz) -> d_(xy) -> d_(x^2 - y^2) -> d_(z^2)#
When half-filled, the d-orbitals will how a total of
Technically, five. (
Explanation:
Here is an illustration of how you can fill up the d-orbitals. The orbitals
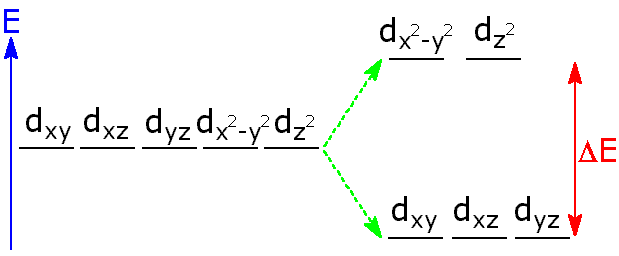
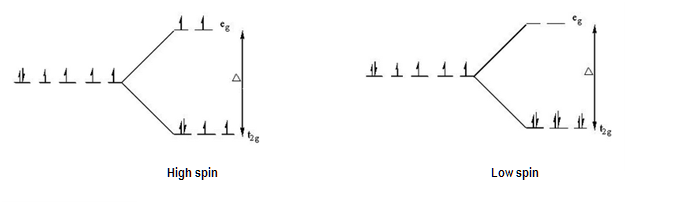
According to the Crystal Field Theory, you can fill these orbitals up either by using high spin or low spin arrangement, depending on the geometry of the molecule (usually, the molecule has a metal element as central atom).