Given a jar of gas (fixed volume and pressure) at 273.15K, the temperature is lowered to 0K, what happens to the volume?
1 Answer
Nov 2, 2015
The volume remains the same
Explanation:
Charles' Law, or the law of volumes, was found in 1787 by Jacques Charles. It states that, for a given mass of an ideal gas at constant pressure, the volume is directly proportional to its absolute temperature, assuming in a closed system.
As a mathematical equation, Charles' Law is written as
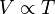
![![enter image source here]](https://useruploads.socratic.org/JIGa3DRybR9IG8XkAabP_5da6ef7dcdf2b0905c87ff58693a7cd8.png)
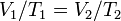
Now solve;
So since you haven't provided a volume initial i cant give u the exact number
But we do know that with a decrease in temp. the volume will decrease and vice versa
But here there is no piston to keep the volume down

