Question #7b5a1
1 Answer
Explanation:
The key to this problem is the fact that the pressure and temperature of the gas sample remain constant before and after the chemical reaction takes place.
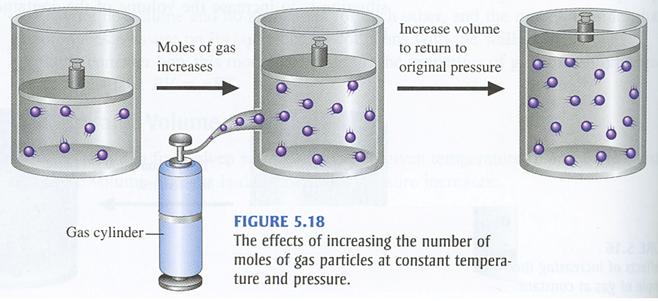
This means that you can use Avogadro's Law, which states that number of moles and volume have a direct relationship when temperature and pressure are kept constant.
In simple terms, when the number of moles of gas increases, the volume of the gas increases as well. Likewise, when the number of moles of gas decreases, the volume of the gas decreases as well.
Mathematically, this is written as
#color(blue)(V_1/n_1 = V_2/n_2)" "# , where
In your case, you know that you initially had
#n_2 = 0.11 + 0.58 = "0.69 moles"#
This means that the new volume of the cylinder will be
#V_2 = n_2/n_1 * V_1#
#V_2 = (0.69color(red)(cancel(color(black)("moles"))))/(0.11color(red)(cancel(color(black)("moles")))) * "2.1 L"#
#V_2 = "13.17 L"#
Rounded to two sig figs, the number of sig figs you have for the values given, the answer will be
#V_2 = color(green)("13 L")#

