What is the shape of SF4 (including bond angles)?
1 Answer
Within the context of VSEPR theory, you can count electrons to determine the electron geometry ("parent" geometry).
Sulfur: 6 valence electrons
Fluorine: 7x4 valence electrons
Total: 34 valence electrons
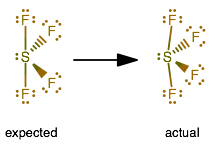
You can put sulfur in the middle because fluorine tends to make single bonds. Therefore, you can put 6x4 on each fluorine, 2x4 to account for four single bonds, and 2 for the last 2 valence electrons available.
As a result, you have 5 electron groups, so the electron geometry would be trigonal bipyramidal. With one lone pair of valence electrons, you get a seesaw molecular geometry.
Note though that the structure is distorted a bit due to the repulsive forces of the lone pair of electrons you see (not bonded).
So, that bends the axial fluorines together a bit. Normally the axial