What would a Bohr Model for magnesium look like?
1 Answer
Dec 17, 2015
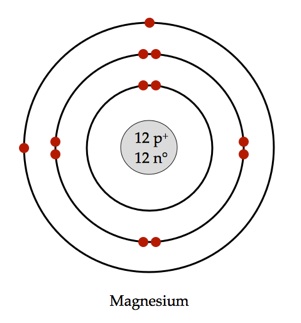
Magnesium has
The first electron shell of a Bohr model holds
The second holds
So far,
The remaining

Magnesium has
The first electron shell of a Bohr model holds
The second holds
So far,
The remaining

